"Ich weiß keinen Zweig der heutigen Naturwissenschaften, der derartig viele und verschiedenartige Interessenkreise berührt, wie die Kolloidchemie. Gewiß, auch Atomtheorie und Radioaktivität interessieren heute jeden intellektuell wachen Menschen. Aber dies sind geistige Delikatessen verglichen mit der Kolloidchemie, die für viele Theoretische und praktische Gebiete nötig ist heute wie das liebe Brot." Wolfgang Ostwald, 1922
In 1861 Thomas Graham coined the term colloid (which means "glue" in Greek) to describe Selmi's "pseudosolutions". The term emphasizes their low rate of diffusion and lack of crystallinity. Graham deduced that the low rate of diffusion implied that the particles were fairly large - at least 1nm in diameter in modern terms. On the other hand, the failure of particle sedimentation implied an upper size limit of 1 micrometer. Graham's definition of the range of particle sizes that characterize the colloidal domain is still widely used today.
Today colloid science is the study of systems involving small particles of
one substance suspended in another. Suspensions in liquids form the basis of
a wide variety of systems of scientific and technological importance,
including paints, ceramics, cosmetics, agricultural sprays, detergents,
soils, biological cells, and many food preparations.
Almost every technique and theoretical procedure of modern physics and
chemistry has been and is being applied to the study of colloids so that
even the specialist colloid chemist finds it difficult to remain au fait
with the many ramifications of the subject.
Why are colloids so interesting and important? The answer is given by the following picture (unfortunately in German):
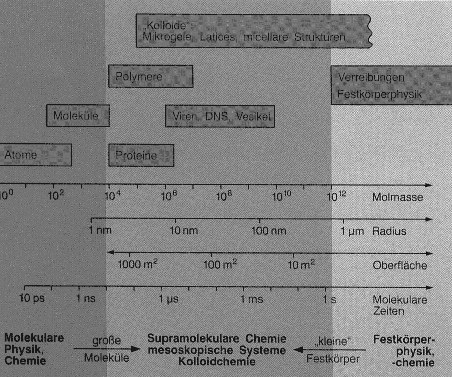
Classical chemistry and physics use relatively small molecules with a
molecular weight up to 10000. Polymer chemistry is using molecules with a
molecular weight three orders of magnitude higher.
On the other hand, solid state physics are interested in the properties of
condensed matter.
Between these two scientific areas is the world of colloid chemistry. Colloid
chemistry is closing the gap between molecular chemistry and solid state
properties. Colloid chemists may use six orders of magnitude of molecular
weight exclusively, including all the corresponding effects. Some people
name this region as "mesoscopic", since the colloidal region is located
in between well known scientific length scales.
The upper picture is very useful, since it shows a whole scientific research
program. The special properties and possibilities of using colloids depend
on their position in between large molecules and small solids.
For example, large surface areas are associated with the characteristic
size of colloidal particles. It is an intrinsic property of all colloidal
systems. For instance, a typical micellar solution containing 0.1M amphiphile
has ca. 40000 sqr-meters of interfacial area per liter solution.
Over the past forty years, colloid science has undergone something of a
revolution, transforming itself from little more than a collection of
qualitative observations of the macroscopic behaviour of some complex
systems into a discipline with a solid theoretical foundation.
It can now boast a set of concepts which can go a long way towards providing
an understanding of the many strange and interesting behaviour patterns
exhibited by colloidal systems.
Colloid chemists are starting make use of hierarchical building principles
in order to synthesise materials with a hirachy of
structure. It is possible to create new properties or property combinations
by making use of the "molecular team formation" on different structure levels.
Colloids are not only small particles, but they can consist of different
functional and structural molecule
types. Choosing the right architecture, these colloidal systems are able to
solve problems that can not be solved by standard molecular systems.
This principle is not new. Nature has been using it for millions of years.
An impressive example is the structure of our hair. Here, Ceratin molecules
aggregate on many different aggregation levels until the final hair is formed. Natural fibres show
surperior properties compared to man made fibres.
Another example is the our skin. There is no synthetic material that is
so soft, elastic and strong as skin. It consits mostly of water, Collagen,
Hyaluronic acid and Proteoglycan. These components form superstructures
which result in the unique properties of our skin.
Such property combinations are not only useful for materials properties, they
are also useful for electro-optic and transport properties of a material.
A lot of research groups are currently starting to synthesise composite materials of polymers
and colloids. They are trying to introduce order into supramolecular systems
by supramolecular order to the systems by selfassembly and modular
synthesis.
Colloid chemistry is one of the most promising scientific areas of our century.
